Health Ministry orders investigation into unauthorised sales of COVID drugs
The Viet Nam Drug Administration has sent a dispatch to HCM City Health Department to investigate the unauthorised selling of COVID-19 drugs, according to the Health Ministry earlier this week.
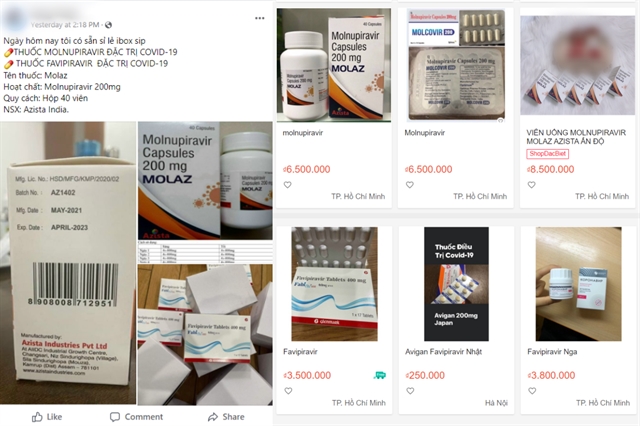 |
| COVID-19 trial drugs are being sold on social media pages and online shopping platforms. — VNS Photo screenshot |
The document was issued after a news article reported that several drugs containing molnupiravir and favipiravir, antiviral substances that are still in clinical trials for COVID-19 treatment, are now being sold on social media and other online platforms.
The medicines have not been licensed for circulation in the country.
The sale and purchase of unapproved drugs is a serious violation of the Law on Pharmacy, posing a threat to people’s health, pandemic prevention and control, as well as increasing the risk of counterfeit and smuggled medicines.
To ensure patient safety, reinforce the 2016 Law on Pharmacy, and address the violations in COVID-19 drug distribution, the Drug Administration has ordered an urgent investigation into the administration and use of drugs containing molnupiravir, which is undergoing clinical trials at medical facilities in HCM City.
Violations must be strictly handled to prevent the smuggling of trial drugs to the market.
At the same time, the city Health Department must also coordinate with the local authorities and departments, including Committee for Prevention of Smuggling, Trade Fraud and Counterfeit Goods and city police forces, to investigate the online advertising, sale and purchase of trial COVID-19 treatment drugs, as well as medicines of unknown origin.
The Drug Administration has also ordered a review on the quantity of treatment kits at COVID-19 treatment facilities.
If a shortage is detected, the city Health Department is required to take necessary measures to address the situation and ensure the supply of medicine for COVID-19 patients.
As of December 7, a total of 42 provinces and cities have been prescribing the antiviral drug molnupiravir for controlled treatment of COVID-19 patients with mild to no symptoms.
This figure has doubled compared to the beginning of November.
To date, the Ministry of Health has administered approximately 250,000 doses of molnupiravir free of charge.
A recent report on the pilot treatment of mild COVID-19 cases, with controlled use of molnupiravir, showed positive results.
Deployed at 22 localities, the programme showed that the drug can significantly reduce viral load, and lower the risk of transmission and severe infection.
The treatment time was also shortened: Patients whose RT-PCR tests returned negative or registered a low viral load increased from 72.1 to 99.1 per cent.
After 14 days of treatment, the rate reaches nearly 100 per cent.
The rate of patients developing severe illness is also very low at 0.02 to 0.06 per cent, with no fatalities.
Source: VNS