Viet Nam's second Covid-19 vaccine candidate enters human trials
A ceremony was held at the Hanoi Medical University on January 21 to kick-start clinical trials of COVIVAC, Viet Nam’s second Covid-19 vaccine candidate and developed based on the new highly-infectious coronavirus variants.
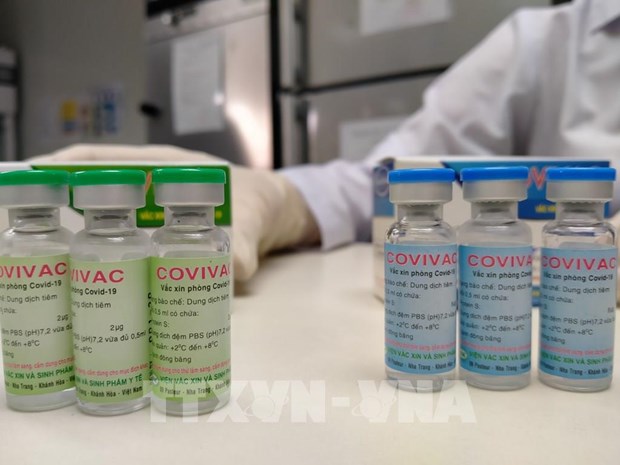 |
| COVIVAC is Viet Nam’s second Covid-19 vaccine candidate and developed based on the new highly-infectious coronavirus variants. (Photo: VNA) |
The home-grown vaccine has been developed by the Nha Trang-based Institute of Vaccines and Medical Biologicals (IVAC) and the Hanoi Medical University since last May, using primary chicken embryo cell culture, a technique the institute used previously to successfully produce seasonal flu vaccines.
COVIVAC has undergone pre-clinical trials in India, the US, and Viet Nam, said IVAC Director Dr Duong Huu Thai, adding that results showed that it satisfies all conditions to be tested on humans.
The vaccine candidate demonstrated high immunogenicity during pre-clinical trials. It was created based on studies of new SARS-CoV-2 strains, according to Dr Pham Van Tac, Director of the Ministry of Health’s Administration of Science Technology and Training.
“We can totally count on this vaccine candidate,” Tac said.
There will be two phases in the trial, with the first conducted on five groups of volunteers totalling 120 people. Each group will be given two shots 28 days apart, with doses of either 1mcg, 3mcg, or 10mcg.
The volunteers, aged 18 to 59, has been recruited by the research team.
The first shot is scheduled to be injected at the Hanoi Medical University in February.
Source: VNA